
If you're in the mood for a little Americana, here's a treat for you, plus a chemistry lesson, which may or may not be.
It doesn't get a whole lot more Americana than Marvin "Popcorn" Sutton, who became famous both for his prowess at making moonshine, and his disdain for federal authorities. Both of these kept Sutton in perpetual trouble with the law, especially for moonshining and bootlegging (1), but he managed to stay out of prison until 2009 when he was convicted of illegal possession of a weapon and a whole lot of untaxed alcohol. Sutton, who committed suicide in 2009, was the subject of a History Channel documentary called "Hillbilly: The Real Story."

(Left) Marvin "Popcorn" Sutton and his still. Photo: Listverse (Right) Sutton's gravestone. You know the word. Photo: wn.com
Buried in this story is a chemistry lesson, which is about a purification technique that many chemists dislike intensely—distillation. Distillation is a process in which liquids are gradually heated in flask or vessel. Since different liquids vaporize at different temperature (this is called the boiling point), as the flask continues to heat, the liquids with the lower boiling point will vaporize first. The vapor passes through a cooling condenser, which converts it back to its liquid form, which is then collected. The distilled liquid is usually purer (but not always) than what was in the fermentation vessel.
Not only is the technique useful for separating mixtures of liquids with different boiling points, but also for removing a liquid, such as alcohol, from a mixture of solids and other liquids. In this case, the crude soup-like material is called a mash—the mixture that causes alcohol to be formed by fermentation. But you'd better do it right, or your customers are going to be mighty sick (or mighty dead) because distillation isn't such a great way of separating liquids that have similar boiling points. Co-distillation—two liquids with different boiling points coming out together is not uncommon, despite the difference in boiling point. This is why when moonshining you'd better "throw away the first cut." (Figure 1).

One of Popcorn Sutton's stills is available on EBay. Probably not free shipping.
When sugars are anaerobically metabolized by yeast, alcohol is formed, but so are byproducts, some of which are nasty. Perhaps the worst is methanol, aka wood alcohol (2). Figure 1 demonstrates the "messiness" of fermentation, the importance of differences in boiling point in distillation, but also some of the limitations of the technique as a method of purification.

Figure 1: Chemical composition of moonshine as a function of distillation temperature. Adapted from Whiskey Still Pro Shop and Stilldragon.org
Here's the rest of the science. The fermentation of alcohol begins with glucose and involves 12 well known biochemical reactions. The first phase, glycolysis consists of a series of ten separate reactions that converts glucose into pyruvic acid, but only in the absence of oxygen. Pyruvic acid then undergoes an enzymatic decarboxylation (this is why gas forms during fermentation - CO2), which converts it to acetaldehyde. Finally, the acetaldehyde is reduced to ethanol by the enzyme alcohol dehydrogenase. Yeast can do a lot of cool things.

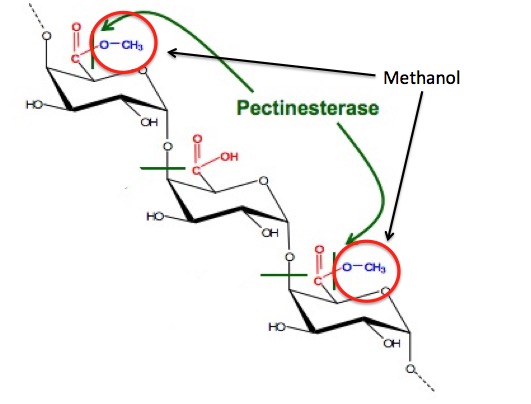
But, where does the methanol in the first cut come from? There is nothing in the fermentation pathway that suggests that it should be there. It turns out that the methanol is formed in an unrelated process—from the methyl ester (red circle) in pectin. Pectin is found in fruit, so when berries, etc. are used as the sugar source, methanol is formed. When molasses is used as the sugar source, methanol is not formed. Traditionally, moonshine is made from corn, which has pectin.
Quiz: Alcohol that is produced from sugars is capable of forming methanol, but usually not very much. Yet there have been many reported cases of fatal methanol poisoning. There are two major ways this can happen. What are they? If you get it right, I will try to coax Council president Hank Campbell to send you a bottle of Jack as a prize, but this is unlikely to happen (3,4).
Good thing that you can now make your own booze. Popcorn would be proud.
Notes:
(1) Moonshine gets its name because it was traditionally made at night to evade law enforcement. The term bootlegger is derived from the practice of transporting alcohol (during times of prohibition) but transporting it in high boots.
(2) A lethal dose of methanol is estimated to be 10-30 mL. The amount of methanol in a package of aspartame is 10 mg. It would take 790 packets of aspartame to equal 10 mL of methanol, yet people are still hysterical about it despite decades of safe use. Enough to make you want to drink.
(3) Alcoholics are disqualified. Sorry.
(4) Hank Campbell is notoriously cheap. So, instead of a bottle of Jack, you will instead get Jack.



