Did you know that 24k gold (at least 99.7% pure) makes lousy jewelry? It is so soft that it can be bent by hand and you can even bite into it. To make it strong enough to be used as jewelry, other metals are added to form alloys (two or more metals dissolved in each other). Silver and copper are the most commonly used metals that are added to gold.
Did you know that the same holds true for pure silver? It is also too soft to be used as jewelry, so it is mixed with copper. The composition of sterling silver is 92.5% silver and 7.5% copper.
Did you know that a silverfish is not made of silver?

OK, even though that last line was untrue, the element silver has enough interesting properties to make a good story. Hopefully, this will be one of them.
Many metals are too chemically reactive to be found in their elemental form in the earth. Instead, they are found as minerals where the element exists in a different chemical form. Silver does both. It can be found as pure silver or in minerals that contain it. Some of these are spectacular (Figure 1). 
Figure 1. Some silver-containing minerals
Other miscellaneous cool stuff about silver...
- Silver salts are highly insoluble in water. (Does anyone know why?) Here is a comparison of silver and sodium salts. Quite a difference.
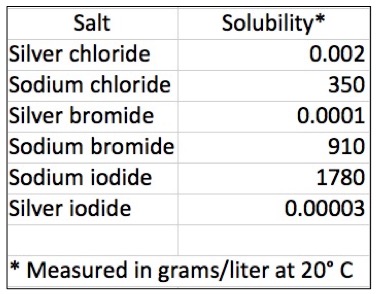
The water solubility of silver vs. sodium halides in grams/liter at 20º C
- This can be shown with a very simple chemical reaction. Silver nitrate is one of the few water-soluble silver salts. If you add any chloride source, the two react immediately to form silver chloride, which participates as a white solid. So what? Here's what.

- Solutions of silver nitrate and sodium chloride are mixed together.
- A white solid, silver chloride, forms instantly.
- The white solid is collected by filtration.

- A template, which covers part of the solid is placed over it.
- A bright light is shined on it for a few minutes.
- The exposed part of the silver chloride has turned purple, while the covered part stays white.
- You have just seen the chemistry of photography.
Photos: YouTube
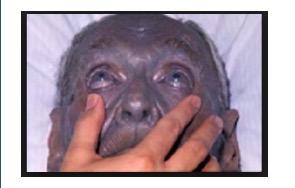
Speaking of purple... Since silver can kill bacteria on surfaces and in wounds (maybe, there is little evidence that it works), a bunch of dopes in the 1800s decided that it was a good idea to swallow some (1). It isn't. The silver accumulates in your skin and turns is purple. The condition is called argyria. And it's irreversible. Yet, you can still find the stuff in vitamin stores.

A 92-year old man with argyria. No longer a fan of grape Popsicles. Photo: Medscape
This does not help with your social life unless your dating demographics include you prefer extinct species:

More:
- Until 1965, dimes, quarters, and half-dollars were made from 90% silver and 10% copper. If you hoarded every silver coin you could get your hands on in 1964 you would be rather wealthy. The value of the metal in one quarter is now $3.00. This is also called the melt value.
- Speaking of coins, pennies and nickels have a smooth edge but dimes, quarters, half-dollars, and silver dollars have a ridged edge. Do you know why? (2)

Examples of ridged-edged (quarters, dimes) and smooth-edged coins (nickels, pennies) (not shown)
And, while we're on the subject, you could check your pocket change in hopes of finding one of these. But don't get your hopes up.

The 1804 silver dollar, one of the rarest of all US coins. This one, which is in outstanding condition, sold for $4.14 million in 1999. (Source: CoinFacts.com). That's quite a bang for a buck.
NOTES:
(1) Silver won't turn you purple unless you swallow the "right" form - colloidal silver, which consists of very small particles of the metal and water.
(2) In the 1700s thieves would shave off a bit of the edge of silver and gold coins and keep the metal. The US Mint decided to make gold and silver coins ridged to discourage this practice. The metal in pennies and nickels was not worth bothering with, so these coins kept their smooth edges.