Lung cancer (LC) is a terrible disease that kills more than 120,000 Americans annually, but there is good news. If LC can be detected while still at a small size before it has invaded and spread, it is curable. And the smaller, the better, as illustrated by the following data. For example, the chance of cure is 92% when less than 1 cm. in diameter (IA1) 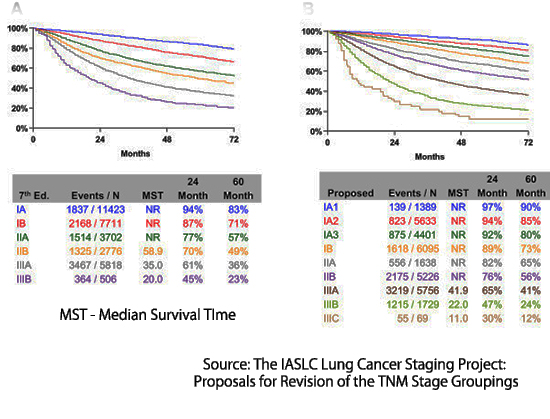 but falls to 73% when beyond 3cm (IB). After the cancer has spread to mediastinal lymph nodes (stage 3) or distant organs (stage 4), the chance of cure drops precipitously.
but falls to 73% when beyond 3cm (IB). After the cancer has spread to mediastinal lymph nodes (stage 3) or distant organs (stage 4), the chance of cure drops precipitously.
The good news is that computerized tomographic screening (CT screening) can detect very small lung cancers below 1 cm in diameter. The bad news is that although CT screening has been covered by private insurance, Medicare, and Medicaid since 2014, participation among those eligible remains very low, and an opportunity to save tens of thousands of lives is being lost.
The Centers for Medicare and Medicaid Services (CMS) agreed to provide first-dollar insurance coverage for CT screening in 2014 and subsequently has extended coverage to approximately fourteen million individuals at increased risk of lung cancer, i.e., smokers between the ages of 50-77, with more than twenty pack-years of exposure, and ex-smokers who have quit smoking within the previous fifteen years.
Despite a decade of CMS coverage, uptake of CT LCS remains low at 18% by various estimates, and an opportunity to prevent thousands of LC deaths has been lost. In recent years, several critics who had long advised against CT LCS or for restricting eligibility are now advocating molecular methods (in which they hold a proprietary interest) as “pre-tests” to determine eligibility for CT scanning.
One such individual is Peter Bach, who now serves as the chief medical officer of Delfi Diagnostics. The company published its initial results in Nature Communications, which subsequently published my critique of the study. DELFI has now published a second article on the use of their molecular “fragmentomics” as a “pre-test” for computerized tomographic lung cancer screening (CT LCS). This prospective case-control study provides stronger evidence than their previous retrospective data, but the paper, concept, and results require scrutiny.
The authors begin by citing the low uptake of CT LCS as an important reason we need a pre-test. They use as an example the case of colon cancer screening, where, despite relatively low sensitivity, molecular screening of both stool and blood misses significant numbers of colon cancer and has found broad appeal and recent regulatory approval.
This argument is unsound. Colonoscopy screening is unpleasant (personal experience); the preparation requires drinking a large volume of an unpalatable liquid, punctuated by frequent urgent trips to the toilet. The following day is lost in travel to an outpatient center, undergoing colonoscopy and recovery from deep intravenous sedation. Additionally, there are risks of complications if biopsy removal of polyps is necessary.
In contradistinction, CT LCS screening requires no need to undress and no IV, just a minute or two spent lying on a padded platform and a few brief breath-holds. Personally, I find this process to be less uncomfortable than a venipuncture.
While the manuscript accurately reports that uptake of CT LCS is low, it does not discuss the role of three authors, Mazzone, Bach, and Silvestri, in contributing to the long delays in screening implementation. These authors recommended against population CT LCS or for limitation of screening for many years, published inaccurate information suggesting that after diagnosis of LC by CT LCS, only one in five would survive, and urged an otherwise healthy woman athlete to “run away” from the CT scanner. They also advised cessation of screening during the COVID pandemic. For these authors to now express disappointment at the current low uptake is cynical at best.
The interpretation of study data is more problematic. As noted earlier, the intent and goal of CT LCS are founded upon the ability of CT scans to detect lung cancer at a very small size. At this point, the chance of metastasis to lymph nodes or distant organs is very low, and cure is achievable. Such CT-identified lung cancers can be treated safely, with low complication rates, using minimally invasive, partial lung resection or radiation therapy, even in elderly patients with co-morbid disease. Survival following treatment remains very high (> 80%), extending to ten or even twenty years. A corollary observation is that there is little utility for a blood test that can detect large or advanced-stage lung cancers. These cancers present with symptoms, after which survival, even with optimal treatment, remains poor.
Does the evidence show that fragmentomics can detect early-stage LC?
The current Delfi study reported the test's sensitivity as 71% in stage 1 patients and much higher percentages in stages 3 and 4. But is a test excluding, at minimum, 29% of patients with lung cancer from subsequent CT screening beneficial or even acceptable? If a positive fragmentomics test were required before a person was eligible for CT LCS, at least 29% of those with lung cancer would go undiagnosed to present later with more advanced, less treatable, symptomatic disease.
Furthermore, the 71% sensitivity estimate itself appears to be unrealistic. The supplemental data provides pertinent information. Only 59% of 92 Stage 1 lung cancers were categorized as Stage 1A, with an upper size limit for the tumor of 3 cm. This implies that 41% were larger, with a lower chance of cure. In modern CT LCS programs, the mean diameter of LC detected is below 1.5 cm. Cancers diagnosed during annual repeat screening are typically smaller than 1 cm. in diameter. Given that we have robust information showing progressively better long-term survival with smaller-size lesions within stage 1A, it is critically important that molecular screening tests demonstrate their ability to detect equally small lung cancers.
To date, DELFI has not yet provided data to show that their test can safely be incorporated as a pre-test into a diagnostic algorithm for lung cancer screening.
To make that case, molecular diagnostic companies must simultaneously test a prospective cohort of CT LCS-screened patients to determine the sensitivity of fragmentomics or other molecular tests in patients with CT-screen-detected lung cancer. Fortunately, such a prospective cohort study is scheduled to open in Europe in the immediate future, where 26,000 people in 6 European countries will be screened for lung cancer with CT LCS. Nine thousand participants will simultaneously be tested using the fragmentomics methodology.
This study has the potential to answer the crucial question of what percentage of those at risk for lung cancer who are eligible for insurance coverage would be excluded from CT LCS by a false negative molecular screen. Based on the current study, early Stage 1A disease detection will be far lower than the authors stated goal of 80%, and in a pre-test algorithm, unacceptably exclude many from participation in CT LCS.
To conclude, under the concept that “one picture is worth a thousand words,” I invite the reader to view the image of DELFI test results, illustrating test scores in study subjects with no cancer and with stage 1 lung cancer.
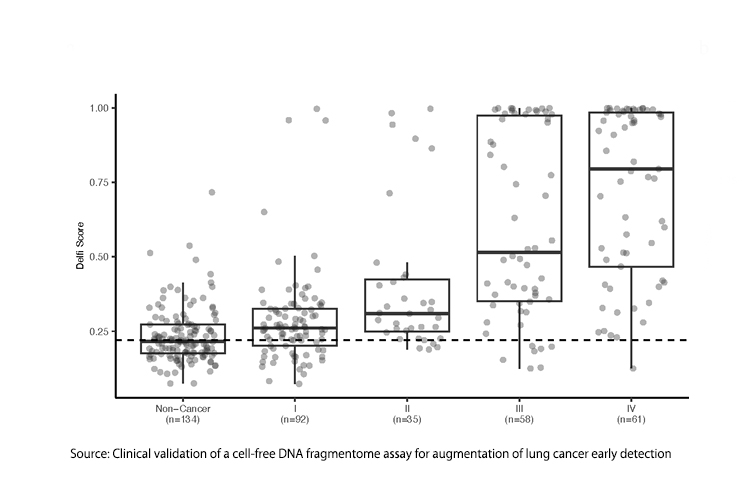
Does fragmentomics really discriminate between those with Stage 1 lung cancer and those with no cancer at all?
CT LCS does a better job. What is the clinical value of a screening test for a more definitive screening test?