It's no secret that droughts have been dominating the news, especially this summer. Even a quick glance at the current NOAA global drought map makes this evident. The lack of rain in two important rice-producing provinces in Chinese has prompted the government to take action to try to protect the crops. How? Cloud seeding. Does it work? Why is an obscure chemical called silver iodide (not silver, as I used in the title) used? Keep reading.

(Left) Global drought map, 8/25/22. China is outlined by a white square. (Right) The Sichuan (white circle) and Hubei (black circle) provinces together account for about 34 million of the 200 million tons of rice produced annually. Source: NOAA
What is cloud seeding?
In short, cloud seeding involves introducing very small amounts of a crystalline substance into clouds. The tiny crystals (also called nuclei) provide a surface area and act as a catalyst, enabling the cold water in the cloud to begin to form crystals of ice. Once that happens, the ice crystals become the catalyst, and the process accelerates (see video below). Rather than go into any more detail, I'll refer you to a nice explanation of how the seeding process works.
Why silver iodide?
Well, that's a damn fine question. Unfortunately, we must disturb your hosts, Steve and Irving, because the only way to explain what's going on is...

Steve (left) and Irving share a tender moment before the lesson.
If you walk into a chemistry lab and pick up a random bottle of chemicals there is roughly a zero percent chance that you'll grab a bottle of silver iodide. It's not routinely found in chem labs. I never used it once in 25 years as a chemist because silver iodide is not generally useful for performing chemical reactions. But the property that makes it of little use in a chemical reaction is the same property that makes it a good seed crystal. It's highly insoluble in water.
- Water solubility
Please don't throw things. To understand why silver iodide is useful for cloud seeding, you must know something about the Pauling electronegativity chart. It's a periodic table that contains an extra number for each element.

A small section of an electronegativity chart.
The number is a measure of electronegativity – how badly atoms want to hang onto (or attract) their electrons. Fluorine, which is the most electronegative element, not only wants to hang to its own electrons but in an extreme case of atomic avarice, also wants to steal others from different elements and is not shy about doing so. This is why Fluorine is the most reactive element. (See Fluorine: the chemical from hell.)
Conversely, metals want to get rid of their electrons. When a metal donates its electrons to a non-metal, both become ions – atoms or molecules having a positive or negative charge. The metal, which got rid of an electron, is now +1. The non-metal, having stolen one, is –1.
When the difference in electronegativity between the two atoms in an inorganic salt (1) is a large number (> 1.5), the resulting salt will be completely ionized, making it polar and soluble in water – a polar solvent (Figure 1).

Figure 1. For metals (left), the lower the value, the higher the reactivity. For non-metals (right), it's the opposite. (Bottom) Sodium iodide (ionic bond) will dissolve in water. Silver iodide (covalent bond) will not.
In the case of sodium iodide, the difference in electronegativity is 1.53, making NaI completely ionized and thus very soluble in water. The result – a molecular Plato's Retreat where there will be non-monogamous sodium and iodide ions cruising around on their own, looking to score with whatever sleazy partners they bump into. Ionized salts tend to have marital problems.
But with silver iodide, the difference in electronegativity is only 0.49, which means that silver and iodine are quite happy together (strong bond) and don't feel the need to go ion swapping like the coked-up swingers in the next beaker. The result is that silver iodide is highly insoluble in water and just sits on the bottom, essentially inert.
The difference in water solubility between the two salts – 614,000,000-fold – makes this clear.
- Sodium iodide 1,842 grams per liter
- Silver iodide 0.000003 grams per liter
To put this in perspective, while 1,842 grams (4 pounds) of sodium iodide will dissolve in one liter (about a quart), it will take 16 million gallons of water (24 Olympic swimming pools) to dissolve the same amount of silver iodide. This is why it is safe to dump it out of planes. What little is used won't dissolve inside the cloud or on the ground where it could theoretically (but does not) contaminate groundwater. Silver iodide is not considered to be a threat to the environment.
It's not just solubility
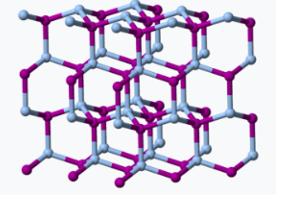
If all that was required for a seeding chemical was insolubility, you could dump all kinds of crap into a cloud. But ice has a similar 3D crystal lattice to sodium iodide.

The crystal lattice structures of silver iodide (Left) and ice (Right) are surprisingly similar. Sources: Snow crystals.com Cal Tech), Wikipedia. Here's a video of what happens when a seed crystal is added to a concentrated solution of the same chemical (2). Had a different chemical been used as a seed, the following would probably not occur.
So, there's all you ever wanted to know (probably much more) about cloud seeding, electronegativity, ionic and covalent bonds, and crystallization. Aside from this method, the only methods to make rain are unverified: 1) buy really expensive tickets to an outdoor concert. And 2) forget your umbrella. That usually helps.
NOTE:
(1) Inorganic in this case means not containing carbon. A salt (M+ X–) is the result of a reaction between an acid and a base or metal with a non-metal. More or less.
(2) Dry ice is also used for cloud seeding.