The opioid crisis, born out of governmental incompetence, has propelled two drugs, naltrexone and naloxone, into the forefront of American consciousness. It is important to know how each of these drugs is used to mitigate opioid overdose and addiction. Despite the similarity of the names (and chemical structures), both drugs have a different function in the body because of how quickly they take to work and how long they last.
NALOXONE
Naloxone (Narcan, Evzio) is the now-famous drug used to reverse opioid overdoses. Its primary use was to reverse heroin overdoses, that is, until fentanyl came along (1). The drug works because it has a greater affinity for the opioid receptors in the brain than drugs like heroin (2), morphine, or oxycodone and rapidly dislodges them from the binding site and reverses the effect of the drug. This is why the drug naloxone saves lives.
Drugs like morphine are opioid receptor agonists – molecules that bind to a receptor on the outside of cells and elicit a pharmacological response on the inside. But naloxone is an opioid antagonist. Antagonists bind to the receptor without eliciting a pharmacological response. Naloxone "sticks" to the receptor and prevents other opioids from binding. (Figure 1). Naloxone is fast-acting (onset time 1-5 minutes) and short-lived, with a half-life of 30-80 minutes.
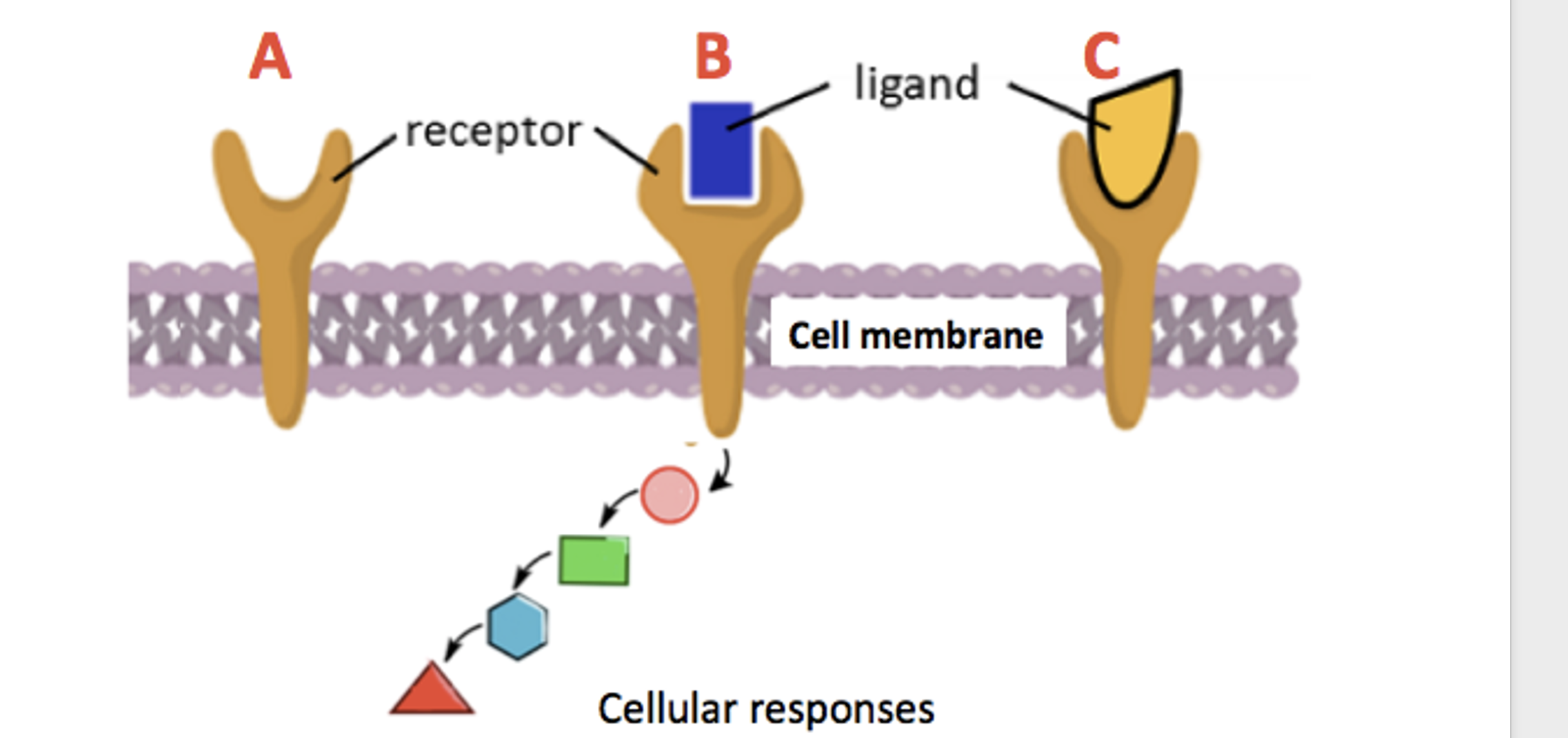
Figure 1. Agonists vs. antagonists. (A) An unoccupied opioid receptor embedded in a cellular membrane. (B) An opioid receptor is bound to a natural agonist, for example, morphine. The drug binds causing a signal (colored shapes) to be sent to the inside of the cell, triggering a series of events leading to pharmacological responses, for example, pain relief. (C) Naloxone, an antagonist, binds to the same receptor but does not send a signal into the cell. Like naloxone, it also blocks opioid agonists from binding. Original figure: Study.com
NALTREXONE
The pharmacology of naltrexone (Vivitrol, ReVia) is different from that of naloxone, even though they are both opioid antagonists. The differences between the two are the rate of onset and half-life/metabolism.
Naloxone rapidly reverses opioid overdoses within minutes, making it a crucial opioid rescue drug. In contrast, naltrexone's action is delayed, precluding its use for rescue therapy. The disparity between the two drugs is striking: while naloxone acts swiftly, naltrexone's onset takes several hours, with effects lasting up to 30 days. Consequently, naltrexone isn't employed for overdose reversal; rather, it serves to block cravings for opioids and potentially alcohol. Acting as a 'slow version' of naloxone, naltrexone occupies opioid receptors post-opioid exposure, hindering the effect of subsequent opioids such as morphine and others. Although the opioid still reaches the brain, naltrexone prevents it from activating its normal target receptor, thereby eliminating the rewarding response associated with drug use. As a result, cravings diminish once the drug's reward is nullified.
HOW SIMILAR ARE THEY CHEMICALLY?
Very. Figure 2 demonstrates that even a minute change in the groups bound to nitrogen – ethene to cyclopropane – has a profound impact on the drug's properties and, thus, its use.
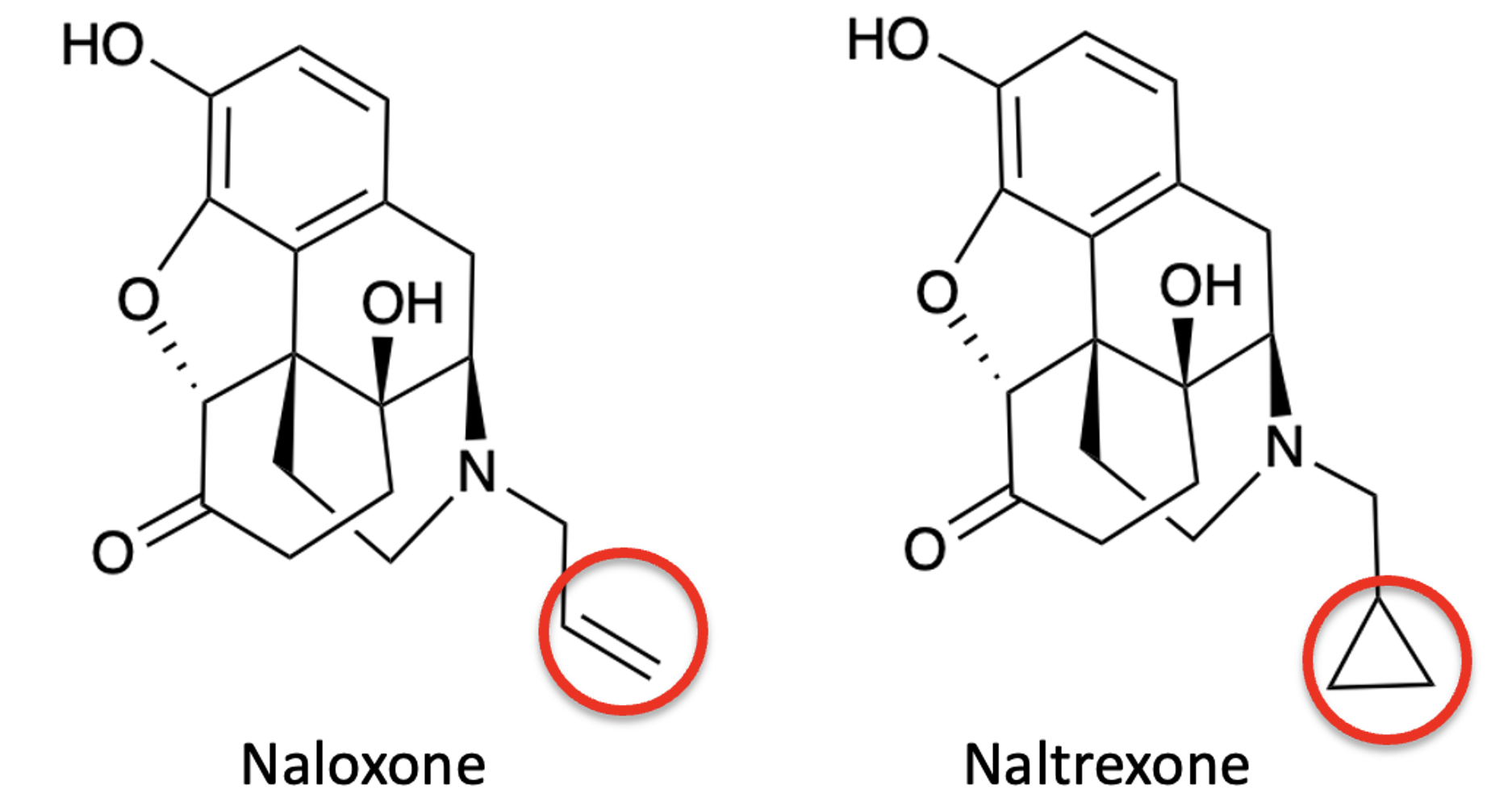
Figure 2. Replacing the ethylene (vinyl) group of naloxone with a cyclopropyl ring forms naltrexone, which has few of its properties.
But the two are even closer than that.
In the world of drug discovery sometimes a carbon-carbon double (ethene) bond can be a liability in a potential drug, for example, a site for oxidation or alkylation. The ethylene and cyclopropyl groups are called bioisosteres – chemical or molecular groups that have similar physical or chemical properties and produce similar biological effects. Using bioisosteres to address undesired properties in a potential drug is an intricate part of the drug discovery process. Here's an example.
Figure 3 shows two nearly identical (3) inhibitors of hepatitis C. Like naloxone and naltrexone, they differ only by the ethylene to cyclopropyl change. The structure on the left was not a viable candidate because the double bond was chemically reactive, which is bad news. Chemically reactive drugs are almost always toxic since they irreversibly bind to proteins and other biomolecules.
For example, chemists at Bristol-Myers-Squibb overcame this potential toxicity by replacing the double bond with a cyclopropyl group, which resulted in a stable inhibitor of one of the key enzymes required for hepatitis C replication (Figure 3).
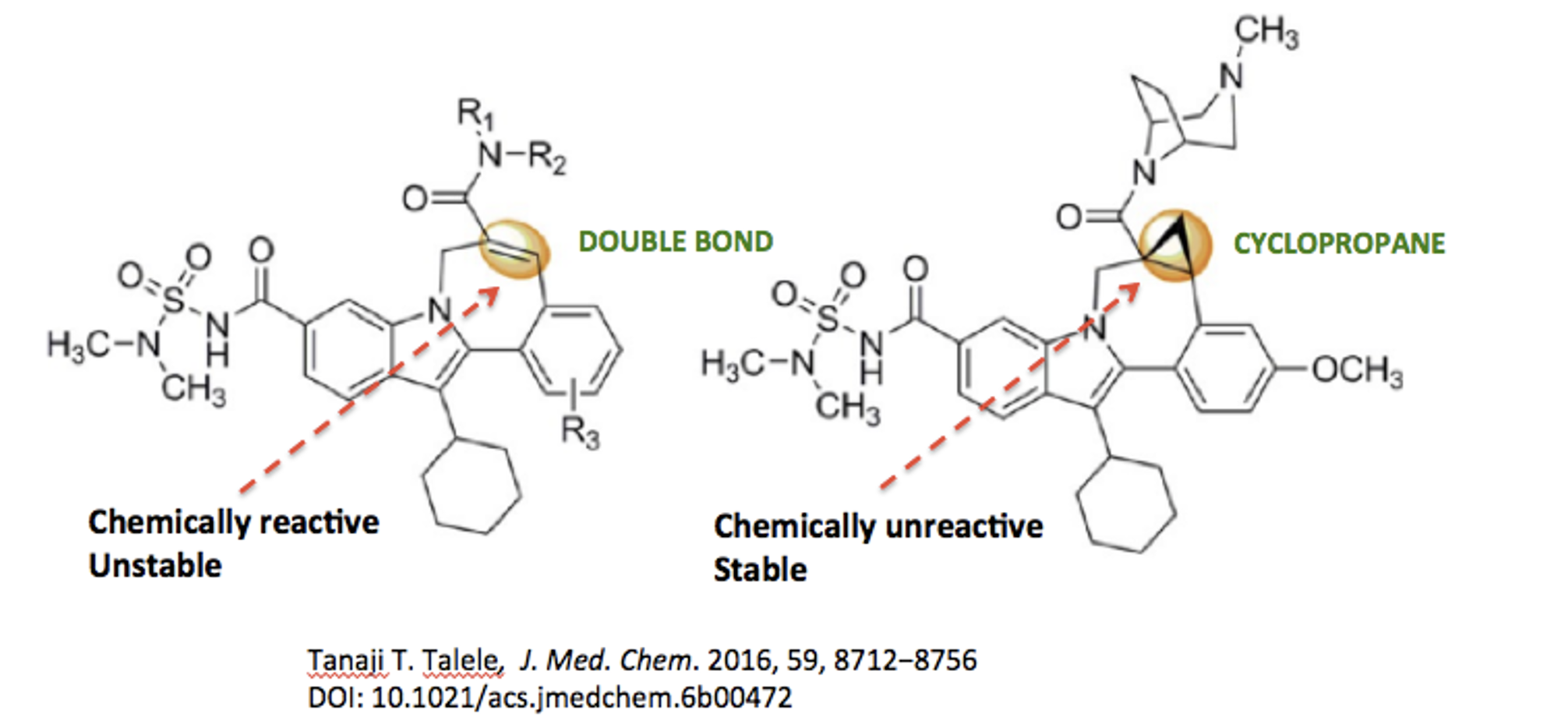
Figure 3. The replacement of a carbon-carbon double bond with a cyclopropane ring resulted in increased stability of a potential hepatitis C drug.
So, two different drugs that are used to combat opioid abuse are used for different purposes, even though, on the surface, they seem to be indistinguishable. But when organic chemistry and metabolism are applied, it begins to make sense.
NOTES:
(1) Naloxone is less effective in saving people who have overdosed on fentanyl because fentanyl binds more tightly than heroin to opioid receptors in the brain, so it is more difficult for naloxone to displace it. Multiple doses are frequently needed, prompting researchers to look for alternatives to naloxone.
“Naloxone seemed to be great for the older opioids, but now that we’re encountering these nonmedical, ungodly [opioids] like carfentanil… we need to get with the times.”
Dr. Jay Kuchera, Florida Resolute Pain Solutions, Stat News April 2018
(2) Heroin does not make it to the brain. In the blood, it is rapidly converted to 6-acetylmorphine and later to morphine. Both are opioid agonists.
(3) It is no accident that cyclopropyl and ethylene groups can sometimes be used interchangeably. Because of size and bonding angle, they are very close in chemical properties.



